Understanding Analytical Method Validation: A Key to Reliable Results
In the world of pharmaceuticals, analytical methods serve as the backbone of accurate testing and data interpretation. However, for these methods to provide reliable and reproducible results, they must be validated. Analytical method validation is a critical process in ensuring that a given method performs as expected, under defined conditions, and with reproducibility across different laboratories and equipment. This blog delves into the importance of analytical method validation, its key components, and Onyx’s approach to it.
What is Analytical Method Validation?
Analytical method validation is a process that provides evidence that an analytical procedure used for testing meets its intended purpose and provides accurate, reliable, and reproducible results. The purpose is to demonstrate that the method is fit for its intended use, whether it’s for identifying a substance, quantifying its concentration, or determining its purity.
For a method to be validated, it must undergo a series of rigorous tests that assess its performance under different conditions. This process involves verifying a variety of parameters, such as accuracy, precision, specificity, and sensitivity, among others. Organisations such as the ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use), EP (European Pharmacopeia) and USP (United States Pharmacopeia) have defined standards and guidelines to ensure that method validation is done comprehensively.
Why is Analytical Method Validation Important?
- Ensures Data Integrity: A validated method ensures that the results generated are consistent, accurate, and reproducible, which is crucial for any scientific or regulatory decision-making process.
- Compliance with Regulatory Standards: Many industries, such as pharmaceuticals and food, are regulated by agencies that require validated methods to ensure safety and quality. Non-compliance could result in costly fines, product recalls, and loss of credibility.
- Protects Consumers: For industries such as food and drugs, where safety is a priority, validated methods guarantee that the products being sold are tested for contaminants, quality, and effectiveness, thus ensuring consumer protection.
- Minimizes Errors: A validated method identifies potential sources of error in the testing process, making it easier to minimize mistakes and deviations from the standard.
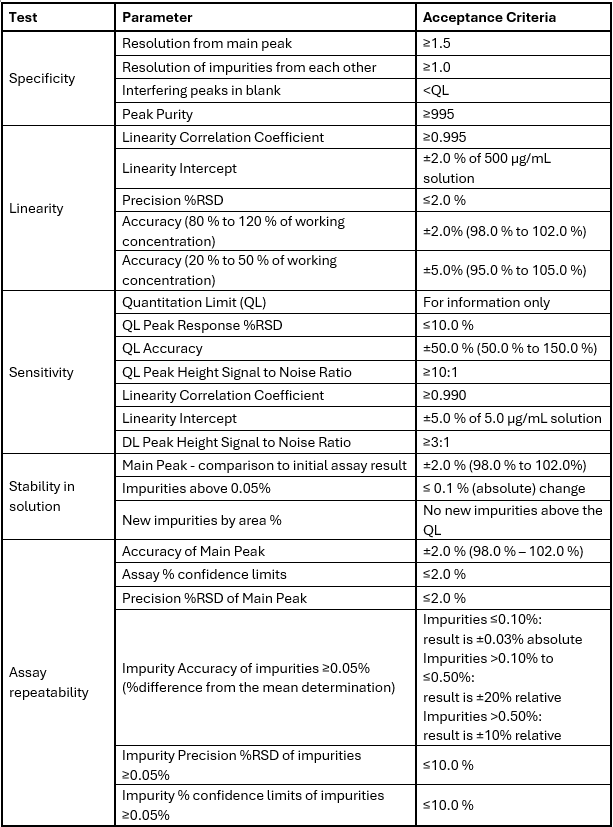
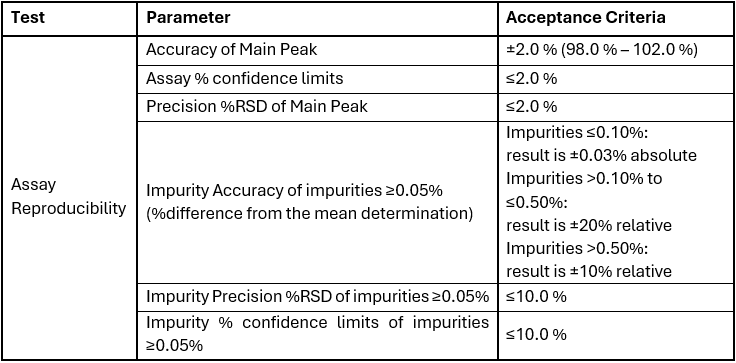
Key Parameters in Analytical Method Validation
A validated analytical method must meet a variety of criteria. The exact performance parameters tested during method validation will change depending on the purpose of the method. Typical core parameters that need to be assessed for a phase 1 method validation process are:
Accuracy
Accuracy refers to the closeness of the test results to the true value or a known standard. A method is considered accurate if it can consistently produce results that are close to the actual value, even in the presence of various interferences.
Precision
Precision refers to the reproducibility of results when the same sample is analysed multiple times under the same conditions. It can be broken down into:
- Repeatability: Precision within a single analytical run.
- Intermediate Precision: Variability over time or between different analysts/equipment.
- Reproducibility: Variability under different laboratories or conditions.
Specificity
Specificity indicates how well the method can distinguish the analyte of interest from other substances that may be present in the sample, including potential interferences and process impurities. Impurities included in the specificity testing are identified and informed by a combination of the process development and forced degradation studies.
Sensitivity (Detection Limit and Quantification Limit)
Sensitivity measures the method’s ability to detect small amounts of the analyte. The detection limit (DL) is the smallest concentration that can be reliably distinguished from the absence of the analyte, while the quantification limit (QL) is the smallest concentration that can be quantified with an acceptable level of accuracy and precision.
Linearity
Linearity assesses the method’s ability to produce results that are directly proportional to the concentration of the analyte over a given range. This range is normally centred around a band between 80% and 120% of the working concentration of the target analyte. This ensures that the method can detect a wide range of analyte concentrations without deviation from a predictable response.
Range
The range defines the upper and lower concentration limits over which the method can provide accurate and precise measurements. A broad range is essential for methods used in various applications with different sample types and concentrations.
Onyx’s approach to Analytical Method Validation
Onyx work on a phase appropriate approach to developing and validating analytical methods, following the guidance set by the ICH under section Q2(R2). The following process describes the typical development and validation of an HPLC method at Phase 1.
Step 1: Method Development and Selection
The first step is choosing the appropriate analytical method based on the specific requirements of the task at hand. This includes understanding the nature of the analyte, the sample matrix and the required sensitivity. During the method development, information and learnings gained from the process and solid-state development teams at Onyx will be considered for further understanding analyte stability and solubility. For more information on Onyx’s approach to HPLC method development, please see the HPLC method development blog.
Step 2: Preliminary Validation and Forced Degradation
Once the method has undergone extensive method development, the analytical development team will subject the method to a preliminary validation, to ensure that the UV response is linear at the identified working concentration. Adequate sensitivity at the QL and DL should also be confirmed, as well as ensuring all specified process impurities and intermediates are all well resolved from the API and each other. The method then undergoes further scrutiny during a forced degradation, using the suggested stress conditions listed in ICH Q1A(R2) and informed by the solid state development to prove that the method is stability indicating ahead of a formal method validation. This study confirms that the method is adequately selective and sensitive to key degradants of the analyte. To learn more about Onyx’s approach to forced degradation studies, please see the forced degradation and stability studies blog.
Step 3: Validation Protocol
Once the method is developed and the preliminary validation has been performed, the validation team drafts a detailed validation protocol that outlines the experiments, conditions, and parameters that will be tested. This protocol defines the scope of the validation, such as the number of trials, sample types, and the limits for each validated parameter.
Step 4: Perform Validation Studies
The validation studies are performed based on the validation protocol. Typically, this involves analysing multiple samples across different concentrations, environmental conditions, and equipment. Each key parameter (accuracy, precision, specificity, etc.) is evaluated against a set criteria.
Step 5: Data Analysis
Once the validation data has been collected, it is analysed to determine whether the method meets the required acceptance criteria. If the method passes the validation tests, the results are documented in a validation report, and the method is considered validated and acceptable to use in a GMP environment. This document serves as a record of the validation process and can be used for regulatory submission or internal quality control.
Other methods validated at Phase 1
At Phase 1, the methods which require validation are the Achiral HPLC, Chiral HPLC (if the API has a chiral centre), residual solvent content and a cleaning verification method, to prove that the API can be sufficiently recovered from steel and glass equipment at the end of the GMP manufacturing process. Onyx utilises a generic validated residual solvents HSGC method that has been validated for a wide variety of solvents, which is supplemented by a recovery check and is appropriate for Phase 1. However, when an API reaches Phase 3, a specific validation is performed to ensure that the specified solvents that used in the process are all sufficiently resolved from one another and to confirm that the compound does not interfere in any way with the solvents. Other compendial method verifications, such as water content by Karl Fischer and Residue on Ignition, are not required to be validated at Phase 1, due to there being pharmacopeial methods in the relevant chapters of the USP and EP and the methods will be assessed at a later phase of work.
How validation changes from Phase 1 to Phase 3
As the lifecycle of an API progresses, more is understood about the compound and its impurities/degradants. Further validations are done, these can either be amendments to the original HPLC validation, just to include parameters that are validated at Phase 2 and Phase 3, such as Intermediate Precision, Impurity sensitivity (relative responses for specified impurities is also assessed in this test, and a response factor generated for any impurities that require one), impurity recovery and robustness. However, it is entirely feasible that further method development will need to be done for Phase 3, as such, a full revalidation of the new method would take place assessing further parameters to ensure the method is applicable for Phase 3 manufacture.
Commercial Validations
Ahead of a GMP Validation campaign, all in process and intermediate testing is evaluated, and critical methods are determined. These methods will then all be validated ahead of the GMP campaign beginning. This could result in over 40 methods across a variety of analytical techniques requiring validation, a significant undertaking.
Conclusion
Analytical method validation is a vital process that underpins the reliability and accuracy of test results, ensuring that methods perform consistently and meet regulatory requirements. Whether in pharmaceuticals, food safety, or environmental analysis, validated methods protect consumers and contribute to the advancement of science and industry. Onyx’s approach to method validation gives customers confidence that every piece of data generated for their API is accurate and reliable.
